#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dylan Verden of KIF1A.ORG summarizes newly published KIF1A-related research and highlights progress in rare disease research and therapeutic development.
KIF1A-Related Research
Preferential transport of synaptic vesicles across neuronal branches is regulated by the levels of the anterograde motor UNC-104/KIF1A
If axons are the highways KIF1A drive on, synapses are like the off-ramps and intersections for local deliveries. Synapses are the interface where two neurons communicate with chemical signals; a single neuron may have thousands of synapses, and the selective growth or shrinking of synapses is a core mechanism for learning and memory.
Synaptic pre-vesicles are a KIF1A cargo that contain many factors that can influence synaptic biology. But as KIF1A carries these pre-vesicles, how does it decide whether to take an off-ramp or stay on the highway? In this week’s pre-print*, researchers studied how UNC-104, a KIF1A analogue in C. elegans worms, changes the movement of cargo at synaptic off-ramps.
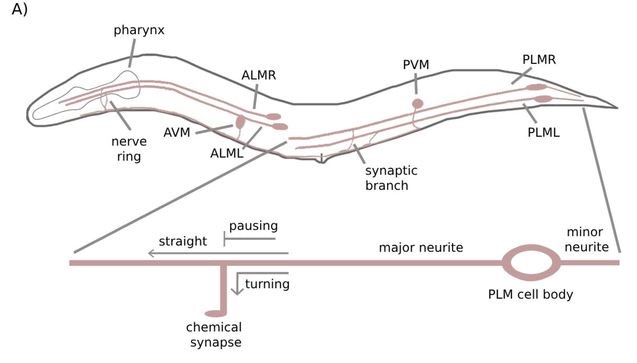
The researchers first established a baseline by measuring how often cargo at intersections move straight ahead, turn toward the synapse, or pause. Under healthy conditions, pre-vesicles most often turn toward the synapse (40-45%) or pause (40-45%), with only 10% of pre-vesicles staying on the main axonal microtubule. Cargo that paused tended to be moving slower in the first place, which could be explained by having less KIF1A motors carrying it.
In worms with only a single intact copy of UNC-104, 80% of cargo paused at synaptic intersections. The same increase in pausing behavior occurred when the researchers knocked out SAM-4, a protein that facilitates attachment of KIF1A to pre-vesicles. Pre-vesicles moved considerably slower in UNC-104 and SAM-4 mutant. Notably, the altered cargo transport of SAM-4 mutants was reversed by introducing a hyperactive UNC-104; indicating that SAM-4’s regulates pre-vesicles through UNC-104 recruitment.
Overexpressing UNC-104 didn’t increase turning behavior of cargo, but did increase the proportion of cargo that go straight ahead instead of pausing. Together, these findings indicate that KIF1A levels are finely regulated to control cargo movement at neuronal branches. We’re often asked about increasing or decreasing KIF1A levels as therapeutic options – studies like this provide us with more information about the effects of such changes.
*What’s a pre-print? Check out this #ScienceSaturday post to learn more.
Rare Roundup
Gene Therapy Approvals Gain Steam in 2023: Treatments Offer New Hopes for Rare Diseases
Gene therapy is an ever-advancing field, but its application to medicine still faces many hurdles; not only the technical development of gene-editing constructs, but the logistics of administering these treatments safely and effectively. This year has seen a growing set of successes in the rare disease space, with varying gene editing effects and administration routes that add to a suite of potential tools.
Notably, last month the FDA granted accelerated approval to a gene therapy for Duchenne Muscular Dystrophy (DMD), a genetic muscle-wasting disorder that primarily impacts boys. The treatment, called Elevidys, consists of a miniature version of the dystrophin gene that is mutated in DMD, and is administered intravenously. Assessments of clinical benefits are still underway, reflecting the FDA’s commitment to accelerated approval of gene therapies for rare disorders. While Elevidys doesn’t target the nervous system like a therapy for KAND would need to, as an injected therapeutic it does lay the groundwork for internal degenerative disorders.

