#ScienceSaturday posts share exciting scientific developments and educational resources with the KAND community. Each week, Dr. Dominique Lessard and Dr. Dylan Verden of KIF1A.ORG summarize newly published KIF1A-related research and highlight progress in rare disease research and therapeutic development.
KIF1A-Related Research
Association of variants in the KIF1A gene with amyotrophic lateral sclerosis
Diagnosing disease is a tricky endeavor, in part because diseases can be categorized in multiple ways:
- Genetically-defined disorder: A disorder defined by the genetic mutation that causes symptoms.
- Symptom-defined disorder: A disorder defined by the types of symptoms its patients typically experience.
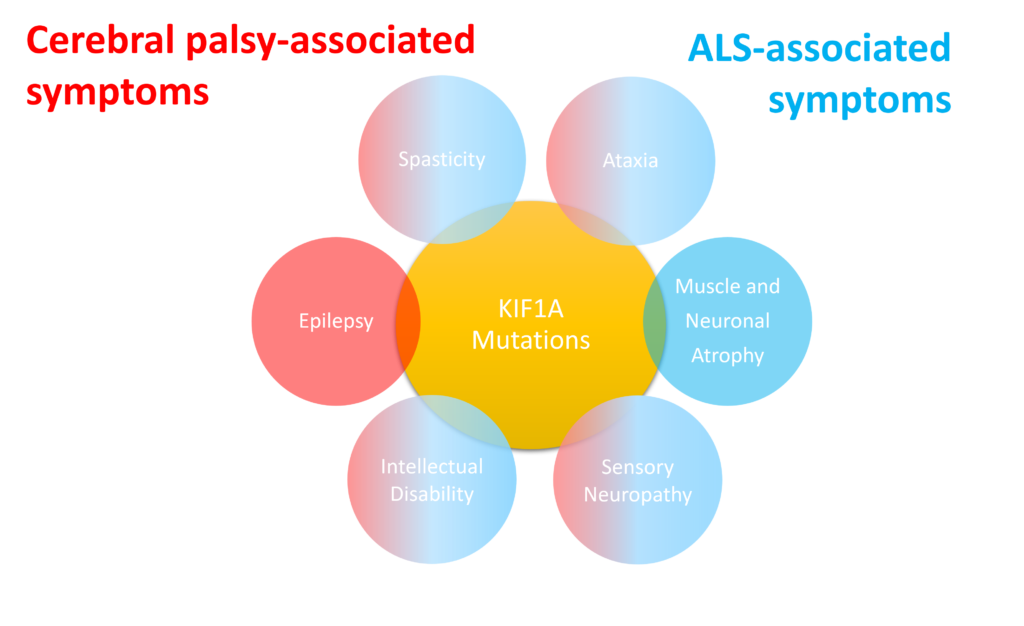
For example, KAND is a genetically-defined disorder caused by KIF1A mutations: Without genetic testing, KAND patients are likely to be diagnosed with a symptom-defined disorder, including cerebral palsy, Rett syndrome, and Charcot-Marie-Tooth disease. This symptom-level approach is also complicated because different people with KAND have different symptoms.
In this week’s article, researchers at Central South University in Changsha, China identified KIF1A mutations in another disease community – Amyotrophic lateral sclerosis, or ALS. ALS is a progressive neurodegenerative disorder that impacts neurons of the central nervous system. Patients experience a wide variety of familiar symptoms including spasticity, ataxia, and muscular atrophy.
There are many genes that contribute to ALS symptoms – we’ve covered KIF5A mutations in ALS in previous #ScienceSaturdays. The researchers analyzed genetic data from multiple databases spanning 8000 patients diagnosed with ALS, and found 45 instances of KIF1A mutations. They focused on a subgroup that has mutations in KIF1A’s cargo-binding domain and self-reported sensory disturbances. These mutations cause KIF1A to hold too tightly to its cargo instead of releasing it at its destination.
So which disease is it and what difference does it make?
It’s worth noting that none of these patients have been formally diagnosed with KAND, but this study highlights an important challenge in diagnosing diseases: A genetically-defined disease can result in many different kinds of symptoms, and a symptom-defined disease can be caused by multiple mutations. It’s important to consider both the genetic cause of the disease and the symptoms experienced:
- Knowing the genetic cause of a disorder is necessary to create gene-based cures for disorders. An ALS patient with a KIF1A mutation can’t take the same gene-based therapy as an ALS patient with a KIF5A mutation.
- Knowing the symptoms of specific patient experiences is necessary for appropriate drug and device-based therapies. A person with a KIF1A mutation that causes sensory neuropathy may benefit from different interventions than a person with spasticity.
Rare Roundup
Gene Treatment for Rare Epilepsy Causes Brain Side Effect in 2 Children
There has been a recent tragedy for two children suffering from KCNT1-related epilepsy who took ASOs for their condition. The ASO, called Valeriasen, reduced Valerie Schenkel’s seizure frequency within weeks. But a year after beginning treatment, doctors detected a buildup of brain fluid, called hydrocephalus, and Valerie passed away on September 5. Hydrocephalus was also detected in Lucy Greenblot, the only other child to take the therapy, but her parents are considering resuming treatment because of the dramatic reduction in seizures.
Gene-based therapies are a new and emerging field that carries risks: There is still much we don’t know about how the ASO, its administration, or the specifics of KCNT1-related epilepsy contributed to hydrocephalus. As we work to develop new therapeutics for KAND, we and our Research Network will continuously gather the best possible information to make these uncertain frontiers as safe as possible. Our hearts go out to the Schenkels.